Novo Nordisk shares in Europe jumped the most in nearly two years after the U.S. FDA approved the Wegovy pill, a once-daily 25 mg oral semaglutide, for long-term weight loss, weight maintenance, and reduction of major adverse cardiovascular events. The approval marks a much-needed win for the struggling Danish pharmaceutical company, which has been hit by market share losses to GLP-1 knockoffs.
“The Wegovy pill is the first oral glucagon-like peptide-1 (GLP-1) receptor agonist therapy approved for weight management,” Novo wrote in a press release earlier on Tuesday.
Approval was based on the Oasis 4 trial, which showed patients taking the daily pill lost an average of 16.6% of body weight. The new pill will be available in the U.S. in early January and will be approved for long-term weight loss and weight maintenance.
BMO Capital Markets analyst Evan David Seigerman told clients the FDA approval gives the company a “much-needed win,” after the “recent challenges maintaining incretin market share dominance.”
Seigerman said that Novo will “benefit from first-mover advantage, capturing patients with a preference for convenience and comfort provided by an oral dosing regimen.” He noted that Eli Lilly’s rival pill, orforglipron, is “just around the corner.”
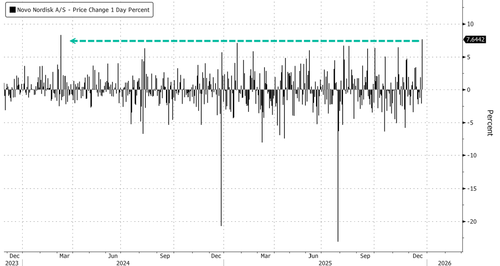
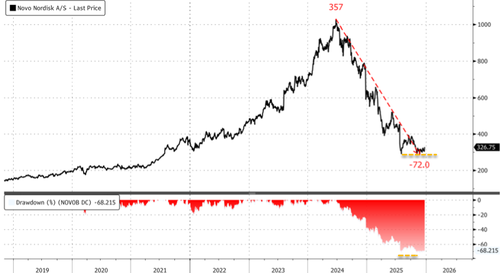
Novo shares in Copenhagen jumped more than 7%, the largest intra-day gain since March 2024. This surge of optimism in the stock comes as market-share losses have pressured it down 48% year to date.
Has a bottom finally formed?
Related:
Will 2026 be a rebound here for Novo?
Loading recommendations…