Just hours after Children’s Hospital Los Angeles halted the use of Sarepta Therapeutics’ Elevidys, the pharma company announced late Monday that it will temporarily suspend all shipments of the gene therapy for Duchenne muscular dystrophy. The move follows three reported deaths linked to the treatment.
“This proactive step will allow Sarepta the necessary time to respond to any requests for information and allow Sarepta and FDA to complete the ELEVIDYS safety labeling supplement process,” Sarepta wrote in a press release, adding, “The Company looks forward to a collaborative, science-driven review process and dialogue with the FDA.”
Sarepta CEO Doug Ingram stated, “The decision to voluntarily and temporarily pause shipments of ELEVIDYS was a painful one, as individuals with Duchenne are losing muscle daily and in need of disease-modifying options.”
“It is important for the patients we serve that Sarepta maintains a productive and positive working relationship with FDA, and it became obvious that maintaining that productive working relationship required this temporary suspension while we address any questions that FDA may have and complete the ELEVIDYS label supplement process,” Ingram said.
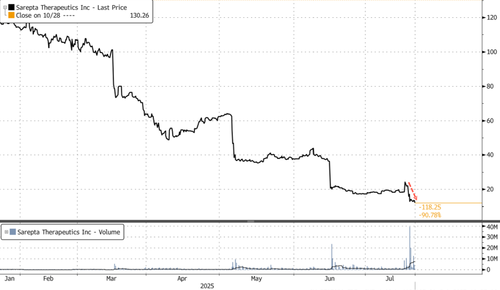
In pre-market trading, shares fell another 4%, compounding yesterday’s 5.36% loss. Year-to-date, the stock is down a staggering 89%, trading at lows not seen since 2015.
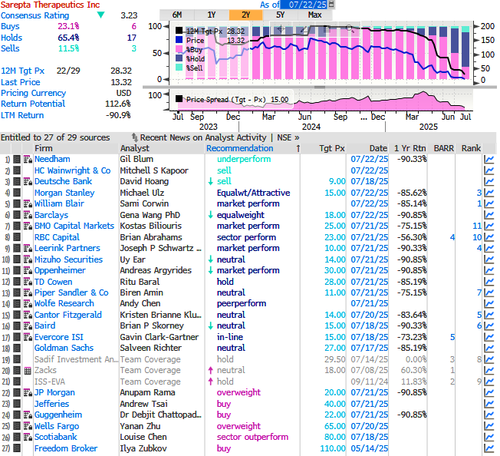
On Monday, HC Wainwright & Co. analyst Mitchell S. Kapoor made the rare move of slashing Sarepta’s price target to zero—from a prior target of $10—while maintaining a sell rating. According to the latest Bloomberg data, there are three sell ratings, 17 holds, and six buys on the stock. The average 12-month price target among Wall Street analysts is $28.32.
Barclays analyst Gena Wang said her desk is stepping to the sidelines due to ongoing regulatory uncertainties, citing “numerous twists and turns” from both the FDA and Sarepta following the death of a third patient. She set a 12-month price target of $18, implying a 35% upside from the last close.
The FDA first asked Sarepta to halt shipments of Elevidys on Friday, following news reports of a third patient death in a clinical trial. The pharma company initially refused, sparking backlash.
Related:
Thanks for playing in the Wall Street casino.
Loading…